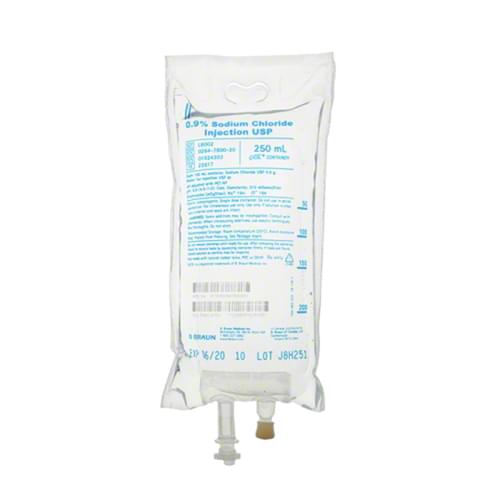
BBraun Sodium Chloride IV Bag – 250ml
$6.42
Category Sodium Chloride IV Bags
Manufacturer Ref BBL8002
- Sterile, nonpyrogenic injection
- For intravenous administration
- Latex, PVC and DEHP free
- 250ml Bag
Categories: Aesthetic Supplies, Sodium Chloride IV Bags
Tag: Sodium Chloride IV Bag
Description
Description
0.9% Sodium Chloride Injection USP is indicated for extracellular fluid replacement, treatment of metabolic alkalosis in the presence of fluid loss and mild sodium depletion.
Details
- Sterile, nonpyrogenic injection
- Without bacteriostatic agents
- For intravenous administration
- Not manufactured with latex
- Does not contain PVC or DEHP
- Specific Gravity: 1.006
Reviews (0)
Be the first to review “BBraun Sodium Chloride IV Bag – 250ml” Cancel reply
Reviews
There are no reviews yet.